Cytokinesis signaling visualized in vitro, using Xenopus cell-free system
Cytokinesis, when two daughter cells are physically separated from one another, is the final stage of cell division. How dividing cells signal where the cleavage furrow should be during cytokinesis has long interested cell biologists. A major stumbling block to probing the underlying mechanisms has been the lack of a cell-free and fully controllable experimental system.
In a new paper appearing in Science (10 October 2014, Science 346 (6206):244-247), Phuong Nguyen and Aaron Groen, along with their colleagues at Harvard Medical School (Boston, MA), have reconstituted the organization of cytokinesis signaling outside living cells, using a the cell-free Xenopus system. The authors examined the biophysics involved in spatial signaling during cytokinesis using powerful imaging techniques, taking advantage of microtubule cytokinesis zones that they assembled on the surface of a microscope slide. These cytokinesis zones are relatively large (~20 mm) in the large Xenopus egg (c. 10x larger than somatic cells) and last for a long duration (>20 mins). This study provides an extraordinary visualization of cleavage furrow and aster formation and, for the first time, reconstitutes this process in an in vitro experimental system.
Adapted from the Editors summary, by Stella M Hurtley
Abstract:
During animal cell division, the cleavage furrow is positioned by microtubules that signal to the actin cortex at the cell midplane. We developed a cell-free system to recapitulate cytokinesis signaling using cytoplasmic extract from Xenopus eggs. Microtubules grew out as asters from artificial centrosomes and met to organize antiparallel overlap zones. These zones blocked the interpenetration of neighboring asters and recruited cytokinesis midzone proteins, including the chromosomal passenger complex (CPC) and centralspindlin. The CPC was transported to overlap zones, which required two motor proteins, Kif4A and a Kif20A paralog. Using supported lipid bilayers to mimic the plasma membrane, we observed the recruitment of cleavage furrow markers, including an active RhoA reporter, at microtubule overlaps. This system opens further approaches to understanding the biophysics of cytokinesis signaling.
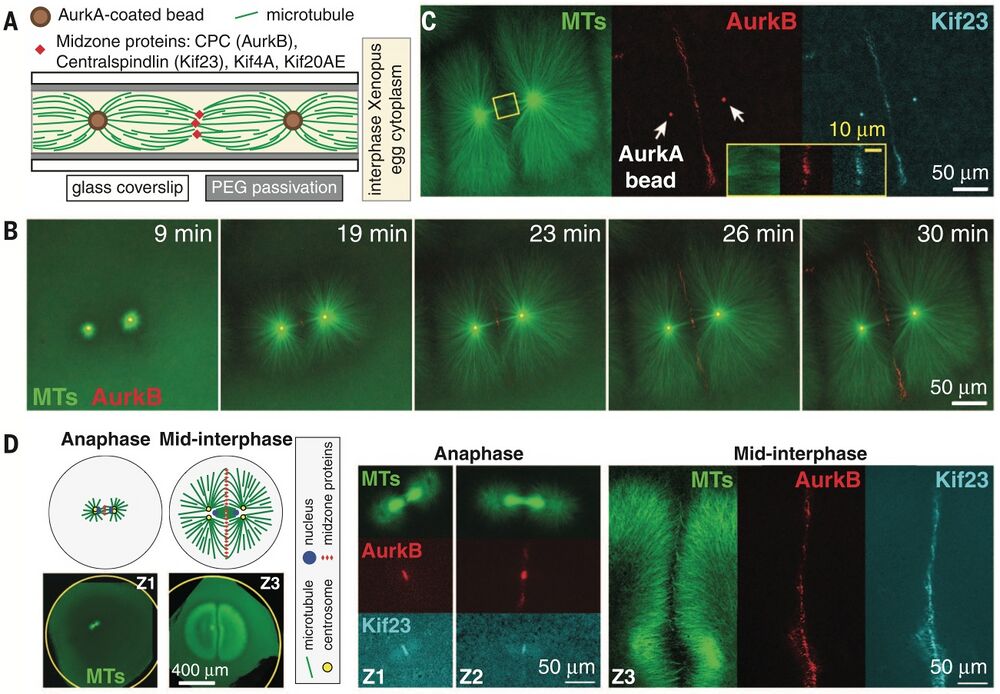
Excerpt from Figure 1, Nguyen et al. - time-lapse images of AurkB recruitment to AurkA coated beads followed by localiation of microtubules.
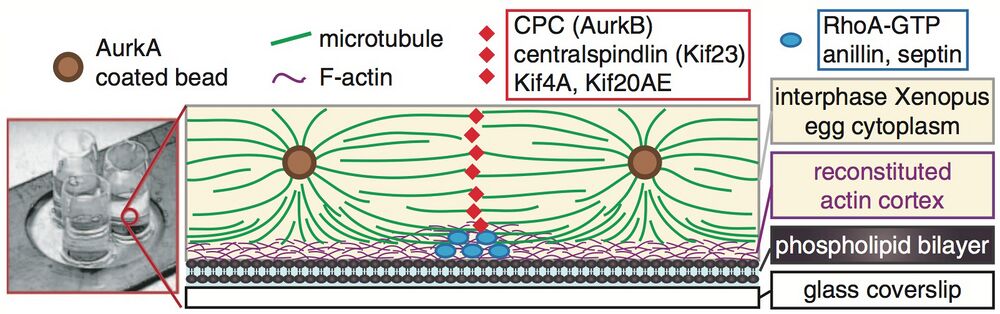
Excerpt from Figure 3, Nguyen et al. - schematic of their experimental setup to visualize localization of a cleavage furrow mimetic.
Spatial organization of cytokinesis signaling reconstituted in a cell-free system. Phuong A. Nguyen et al. Science 346, 244 (2014); DOI: 10.1126/science.1256773
Readers may view, browse, and/or download material for temporary copying purposes only, provided these uses are for noncommercial personal purposes. Except as provided by law, this material may not be further reproduced, distributed, transmitted, modified, adapted, performed, displayed, published, or sold in whole or in part, without prior written permission from the publisher.
Last Updated: 2014-11-07