Cranial placodes in Xenopus development
Cranial placodes:
Placodes are areas of ectoderm that undergo morphogenetic movements including thickening, invagination, and migration. Specification of these cell patches after these initial steps make important contributions to many cranial sensory organs and ganglia.
Nearby cells of the neural crest delaminate, migrate internally and contribute to the neurons and glia of the peripheral nervous system, cranial skeleton, pigment cells, and other organ systems.
A more complex head distinguishes vertebrates from other chordates. Several cranial structures, such as specialized sensory organs and feeding apparatus, evolved in vertebrates which are derived from the cranial placodes and neural crest cells.
The images below highlight the location, migration, and specification of the cranial placodes, the tissues and structures to which they contribute, and key genes expressed during placodal development and specialization.
The majority of the diagrams are taken from publications from the Schlosser lab (see references below).
[click on images below to view enlargement]
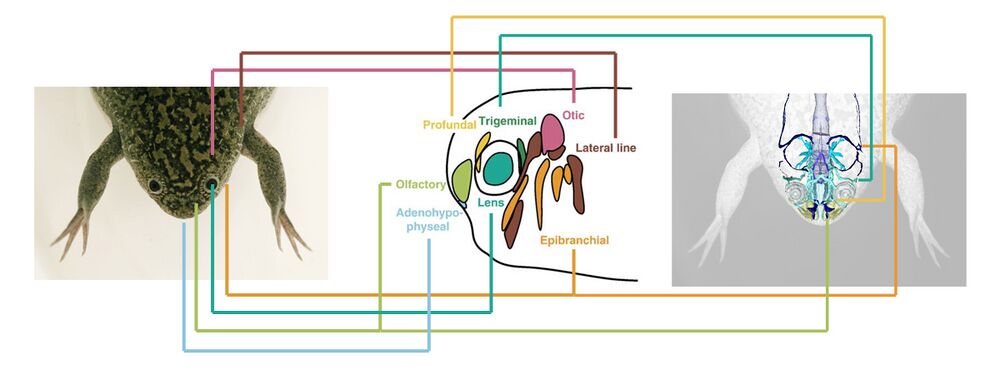
The olfactory placode contributes to the nasal sensory organs. The adenohypo-physeal placode contributes to the mouth and pituitary gland. The lens placode becomes the lens of the eye. The epibranchial placode contributes to cranial nerve X. The lateral line sensory organs derive from the lateral line placode. The ear sensory organ derives from the otic placode. The trigeminal placode contributes to cranial nerve V. The profundal placode contributes to cranial nerve II.
Photo credits: frog - Samuel Brod, EXRC; nervous system - Porro & Richards (2017); placodes - Schlosser (2006)
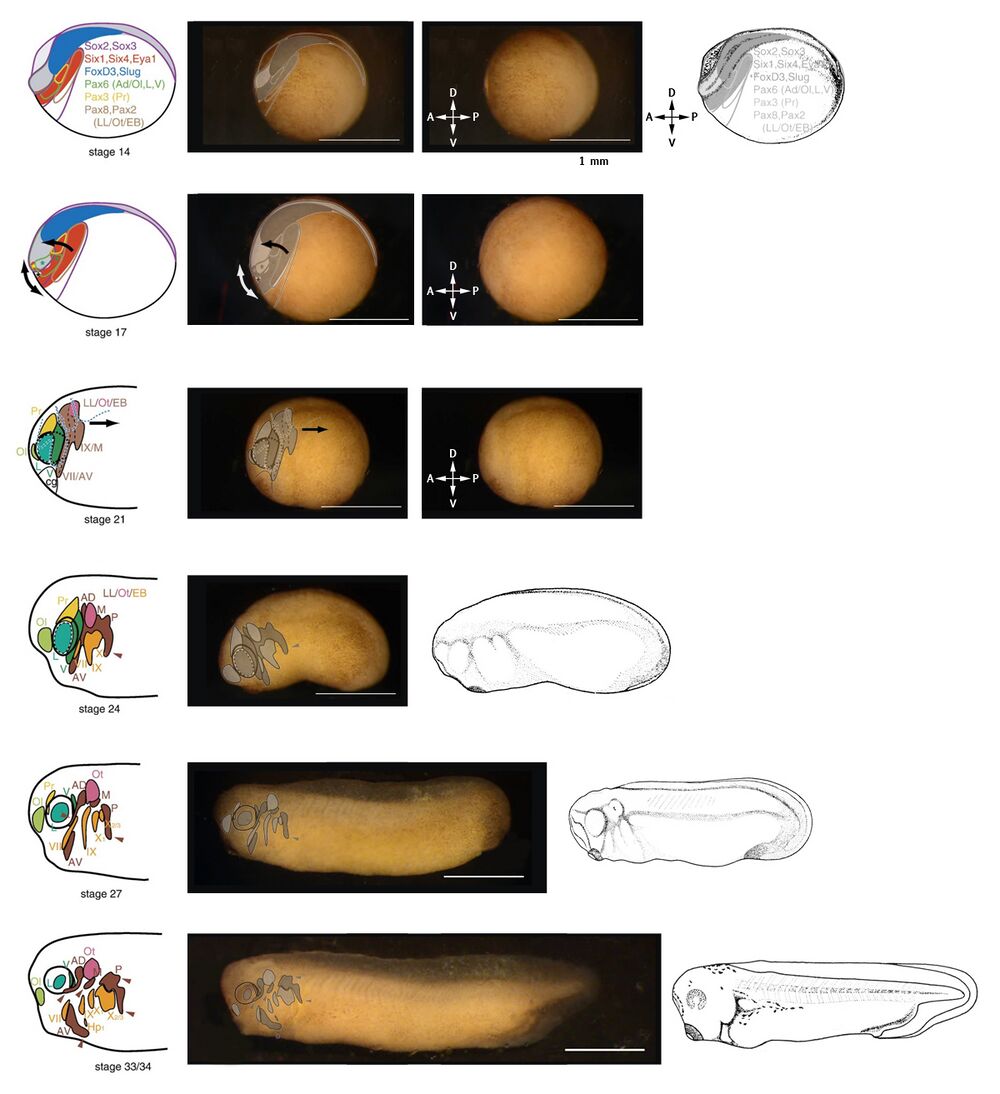
Placodal areas are overlayed on images of embryos to emphasize cellular location, movement, and segregation.
NF stage 14: Lateral view of the embryo at neurula stage. Placodal areas form within the neurectoderm, at and surrounding the neural folds (the anterior of the embryo). Key genes are listed and their expression areas are indicated.
NF stage 17: Morphogenetic movements of the placodal areas follows the closing of the neural folds (top arrow), and the elongation of the head (arrow below).
NF stage 21: Definition and segregation of cranial placodes after neural fold closure. Dorsal to the cement gland (cg), the olfactory (Ol), lens (L), and profundal (Pr) placodes start to differentiate. Posteriorally, the lateral line (LL), otic (Ot), and epibranchial (EB), are not yet separated. Convergent extension of the embryo begins.
NF stage 24 to NF stage 33/34: further separation and definition of the cranial placodes. Convergent extension of the embryo progresses and the embryo grows longer.
Abbreviations: Ad/Ol, anterior placodal area, from which adenohypophysial (Ad) and olfactory placodes (Ol) develop; AV, anteroventral lateral line placode; cg, cement gland; Hp1, first hypobranchial placode; L, prospective lens placode (hatched outline), lens placode or lens (invagination of placode between stages 27 and 33/34); LL/Ot/EB, posterior placodal area, from which lateral line (LL), otic (Ot), and epibranchial (EB) placodes develop; M, middle lateral line placode; Ol, olfactory placode; Ot, otic placode or vesicle (invagination of placode between stages 24 and 33/34); P, posterior lateral line placode; Pr, profundal placode; V, trigeminal placode; VII, facial epibranchial placode; IX, glossopharyngeal epibranchial placode; X1, first vagal epibranchial placode; X2/3, second and third vagal epibranchial placodes (fused).
Image credits: placodal images - Schlosser & Ahrens (2004); embryo images - Davis lab; drawings: Nieuwkoop and Faber (1994).
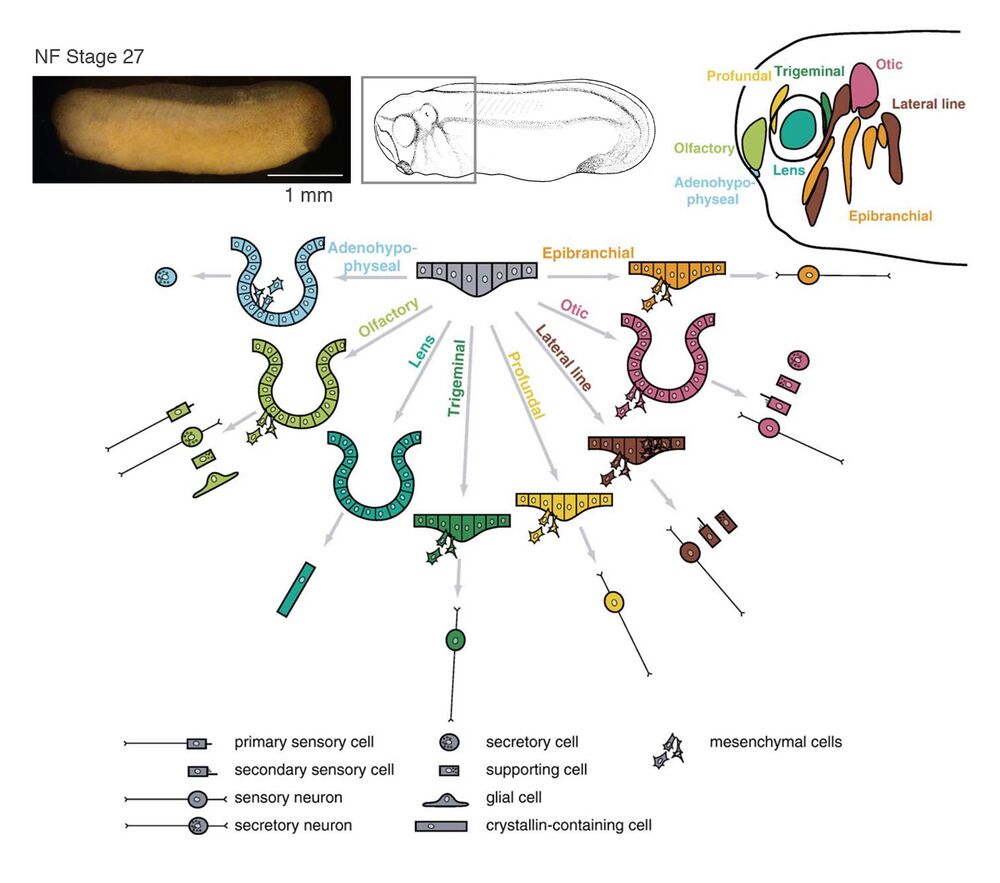
A schematic summary of morphogenesis and cellular derivatives of the various cranial placodes. Invagination occurs in adenohypophyseal, olfactory, lens, and otic placodes. In all placodes except the lens placode, some cells migrate away from the placodal epithelium as mesenchymal cells to form sensory neurons, secretory cells, or glial cells. In lateral line placodes, a subset of cells migrates along the basement membrane to form the lateral line primordia.
Image credits: placodal images - Schlosser (2006); embryo image - Davis lab; drawing: Nieuwkoop and Faber (1994).
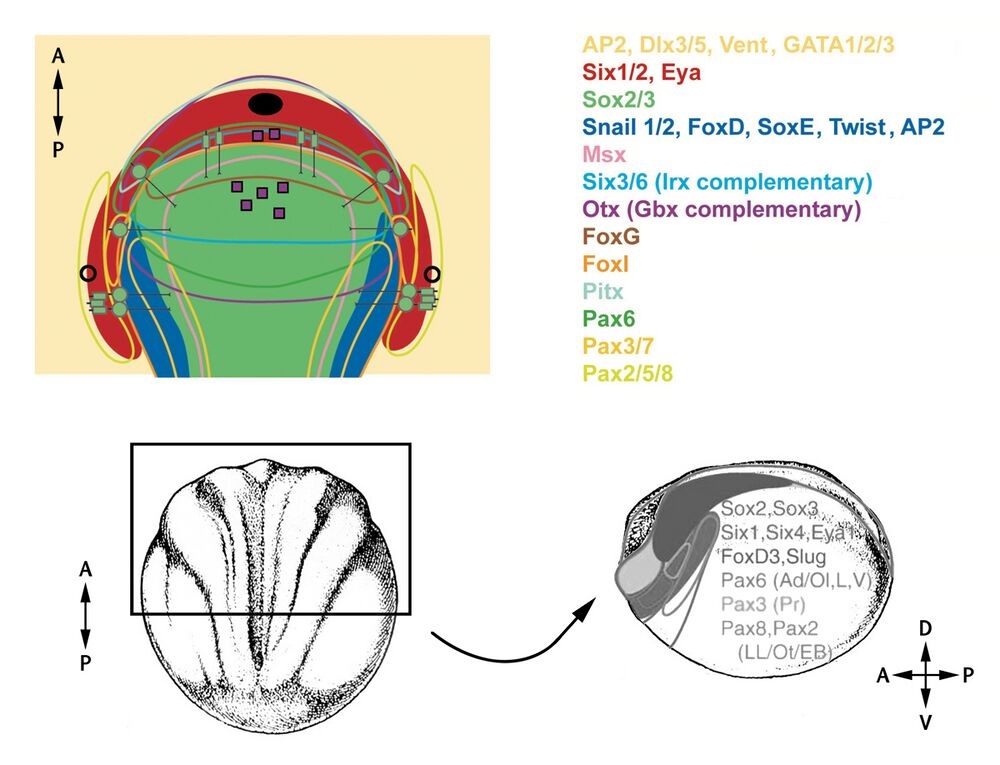
A flattened representation of the dorsal view of anteriodorsal portion of a vertebrate embryo at neurula stage. The neural plate is indicated in green and epidermis in yellow. Domains of Six1/2 and Eya expression are shown in red. Colored outlines enclose transcription factor expression domains except for Msx1 and FoxI, which are expressed peripheral to the lines shown. The neural crest domain which coexpresses Snail1/2 with an array of other transcription factors is shown in solid blue. Expression domains of Irx and Gbx genes are not shown, but are posteriorly abutting the domains of Six3/6 and Otx expression, respectively.
Links to Xenbase gene pages of the genes listed: ap2a1, dlx3, dlx5, ventx1.1, gata1, gata2, gata3, six1, six2, sox2, sox3, snai1, snai2, foxd1, sox8, sox9, sox10, twist1, twist2, msx1, msx2, six3, six6, irx1, otx1, foxg1, foxl1, pitx1, pax6, pax3, pax7, pax2, pax5, and pax8.
Black oval: mouth primordium; black circles: pharyngeal pouch primordia; purple squares: neurosecratory cells; green circle with line: sensory neurons; small green rectangles: secondary sensory cells; small green rectangles with lines: primary sensory cells.
Bottom section: NF stage 14 drawing, dorsal view on the left with anteriodorsal area comparable to the top schematic indicated. Embryo rotated to lateral view on the right. Gene expression domains overlayed.
Image credits: gene expression patterns - Patthey et al. (2014) and Schlosser & Ahrens (2004); drawings: Nieuwkoop and Faber (1994).
References:
Patthey C, Schlosser G, Shimeld SM. The evolutionary history of vertebrate cranial placodes--I: cell type evolution. Dev Biol. 2014 May 1;389(1):82-97.
Schlosser G. Induction and specification of cranial placodes. Dev Biol. 2006 Jun 15;294(2):303-51.
Schlosser G, Ahrens K. Molecular anatomy of placode development in Xenopus laevis. Dev Biol. 2004 Jul 15;271(2):439-66.
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Last Updated: 2019-09-01