Bidirectional multiciliated cell extrusion is controlled by Notch-driven basal extrusion and Piezo1-driven apical extrusion
Rosa Ventrella, Sun K. Kim, Jennifer Sheridan, Aline Grata, Enzo Bresteau, Osama A. Hassan, Eve E. Suva, Peter Walentek, Brian J. Mitchell
Development (2023) 150 (17): dev201612. https://doi.org/10.1242/dev.201612
Click here to view article at Development.
Click here to view article on PubMed.

Time course of Multiciliated cell disappearance from ST38 to ST48 using both the cilia marker acetylate tubulin and the multiciliated cell marker TgTub-MemRFP with DAPI and Phallodin marking all cells.
Summary
All of the multiciliated cells of the Xenopus embryonic skin are lost by Stage 48. Ventrella et al. publish in Development that these cells are extruded from the epithelium using two distinct mechanisms. First, many cells are driven by Notch signaling towards basal extrusion and apoptosis. Cells that evade Notch signaling are driven towards apical extrusion via cell crowding and mechanosensory signaling. This represents a novel form of bi-directional cell extrusion.
Abstract
Xenopus embryos are covered with a complex epithelium containing numerous multiciliated cells (MCCs). During late-stage development, there is a dramatic remodeling of the epithelium that involves the complete loss of MCCs. Cell extrusion is a well-characterized process for driving cell loss while maintaining epithelial barrier function. Normal cell extrusion is typically unidirectional, whereas bidirectional extrusion is often associated with disease (e.g. cancer). We describe two distinct mechanisms for MCC extrusion, a basal extrusion driven by Notch signaling and an apical extrusion driven by Piezo1. Early in the process there is a strong bias towards basal extrusion, but as development continues there is a shift towards apical extrusion. Importantly, response to the Notch signal is age dependent and governed by the maintenance of the MCC transcriptional program such that extension of this program is protective against cell loss. In contrast, later apical extrusion is regulated by Piezo1, such that premature activation of Piezo1 leads to early extrusion while blocking Piezo1 leads to MCC maintenance. Distinct mechanisms for MCC loss underlie the importance of their removal during epithelial remodeling.
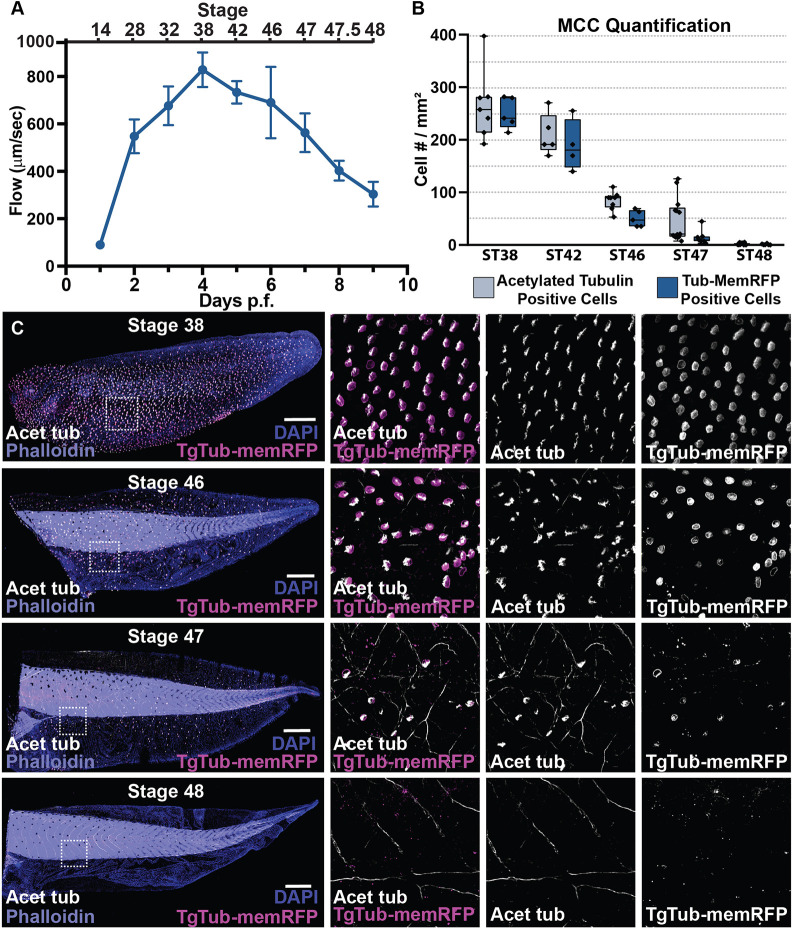
Fig. 1. The loss of cilia-driven fluid flow accompanies the loss of multiciliated cells. (A) Quantification of cilia-driven fluid flow, as measured by fluorescent bead displacement across the surface of the epithelium showing that flow peaks at 4 dpf and is essentially lost by 9 dpf, n=8 animals per time point (data are mean±s.d.). (B) Quantification of MCC number using both the transgenic line TgTub-memRFP driving MCC-specific expression of membrane RFP and antibody staining of acetylated tubulin, n>3 embryos per time point. Box and whiskers plot represents 25th-75th percentiles (boxes), minimum and maximum values (whiskers), with the line representing the median. (C) Representative images of the progression of MCC loss in embryos between ST38 and ST48, showing acetylated Tub (white), TgTub-memRFP (magenta), phalloidin (purple) and DAPI (blue). Scale bars: 500 µm.
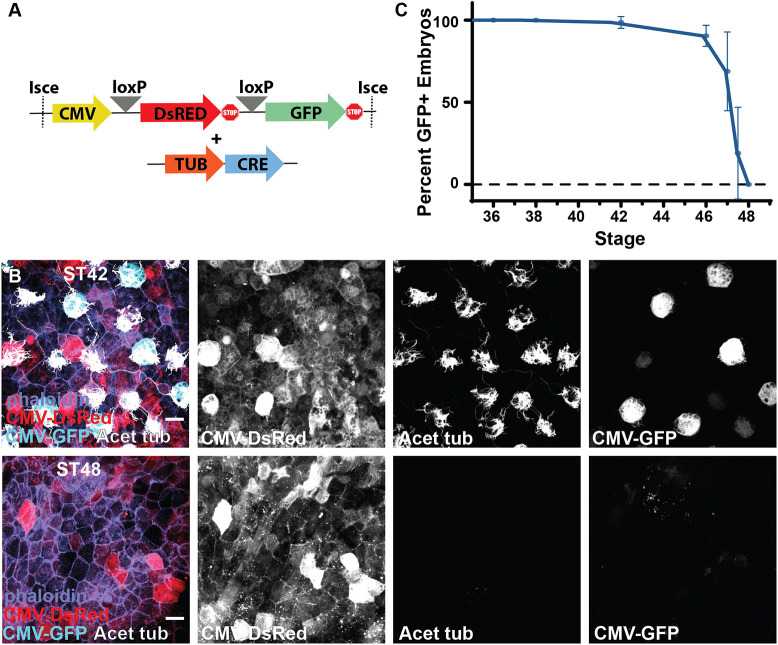
Fig. 2. Lineage tracing of MCC fate. (A) Experimental design showing lineage-tracing constructs of the CMV DsRed loxP GFP cassette together with the Tub-driven CRE. (B) Lineage-tracing experiment showing the conversion of DsRed to GFP in ST42 MCCs upon expression of CRE specifically in MCCs under control of the Tub promoter, and the loss of MCC-specific CMV expression of GFP despite the maintenance of broad CMV-driven DsRed expression at ST48. (C) Developmental quantification of embryos containing GFP-positive cells in lineage-tracing experiments, n=102 transgenic animals over five independent experiments (data are mean±s.d.). Scale bars: 20 µm.
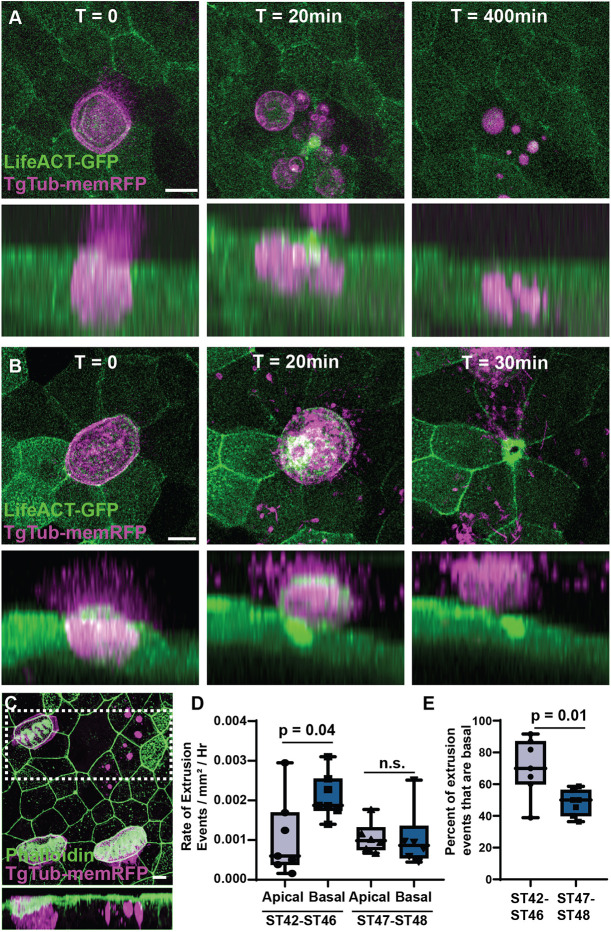
Fig. 3. MCCs are extruded both basally and apically. (A) Representative frames from a time-lapse movie of TgTub-memRFP embryos injected with LifeACT-GFP showing a MCC undergoing basal extrusion with the RFP-positive cell remnants remaining visible for at least 400 min. Lower panels represents a side projection (see Movie 2). (B) Representative frames from a time-lapse movie showing a MCC undergoing apical extrusion. Lower panel represents a side projection (see Movie 3). (C) Representative example of RFP-positive MCC remnants in fixed tissue stained with phalloidin. Lower panel represents a side projection of boxed area. Scale bars: 10 µm. (D) Quantification of the rate of apical and basal extrusion events using long-term light-sheet imaging between ST42 and ST46 (P=0.04), and between ST46 and ST48 (n.s.), n=132 extrusion events from five ST42-ST46 embryos, and 69 events from six embryos post ST46-ST48. (E) Percentage of extrusion events that are basal between ST42 and ST46, and ST46 and ST48 (P=0.01). Box and whiskers plots represent 25th-75th percentiles (boxes), minimum and maximum values (whiskers), with the line representing the median (see Movie 4).
Adapted with permission from The Company of Biologists on behalf of Development: Ventrella et al. (2023). Bidirectional multiciliated cell extrusion is controlled by Notch-driven basal extrusion and Piezo1-driven apical extrusion. Development (2023) 150 (17): dev201612. https://doi.org/10.1242/dev.201612
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
