MC5 Plays Independent Roles in Congenital Heart Disease and Neurodevelopmental Disability
Int J Mol Sci 2023 Dec 28;251:. doi: 10.3390/ijms25010430.
O'Brien MP, Pryzhkova MV, Lake EMR, Mandino F, Shen X, Karnik R, Atkins A, Xu MJ, Ji W, Konstantino M, Brueckner M, Ment LR, Khokha MK, Jordan PW.
Click here to view article at the International Journal of Molecular Sciences.
Click here to view article on PubMed.
Click here to view article on Xenbase.
Abstract
Up to 50% of patients with severe congenital heart disease (CHD) develop life-altering neurodevelopmental disability (NDD). It has been presumed that NDD arises in CHD cases because of hypoxia before, during, or after cardiac surgery. Recent studies detected an enrichment in de novo mutations in CHD and NDD, as well as significant overlap between CHD and NDD candidate genes. However, there is limited evidence demonstrating that genes causing CHD can produce NDD independent of hypoxia. A patient with hypoplastic left heart syndrome and gross motor delay presented with a de novo mutation in SMC5. Modeling mutation of smc5 in Xenopus tropicalis embryos resulted in reduced heart size, decreased brain length, and disrupted pax6 patterning. To evaluate the cardiac development, we induced the conditional knockout (cKO) of Smc5 in mouse cardiomyocytes, which led to the depletion of mature cardiomyocytes and abnormal contractility. To test a role for Smc5 specifically in the brain, we induced cKO in the mouse central nervous system, which resulted in decreased brain volume, and diminished connectivity between areas related to motor function but did not affect vascular or brain ventricular volume. We propose that genetic factors, rather than hypoxia alone, can contribute when NDD and CHD cases occur concurrently.
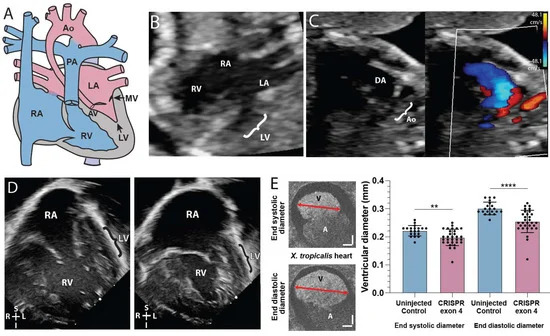
Figure 1. SMC5 variant associated with impaired cardiac development in proband and frog knockdown model. (A) Graphic of hypoplastic left heart syndrome showing the small left ventricle (LV), small stenotic mitral valve (MV), aortic valve (AV), and hypoplastic aorta (Ao). (B) Fetal echocardiogram at 19 weeks gestation in an apical 4-chamber view demonstrates reduced left ventricular (LV) size compared to the right ventricle (RV) as well as the larger right atrium (RA) and small left atrium (LA). (C) A fetal echocardiogram at 23 weeks gestation in a view of the aortic arch demonstrates a small aortic arch (Ao) and larger ductus arteriosus (DA) with color Doppler imaging showing minimal blood flow (red) through the hypoplastic aortic arch and a much larger volume of blood flow (blue) across ductus arteriosus. Color scales estimate fluid velocity, with each color representing fluid flow toward (red) or away (blue) from the ultrasound probe located at the top of the image. (D) Postnatal echocardiogram at 28 days old in an apical 4-chamber view demonstrates a large right atrium (RA) and right ventricle (RV) with a thin hypoplastic left ventricle (LV). (E) Cross section of tadpole atria (A) and ventricle (V) of stage 45 live X. tropicalis embryos (n = 50). Scale bars indicating 50 m. ** p < 0.01, **** p < 0.0001 by t-test in (E).
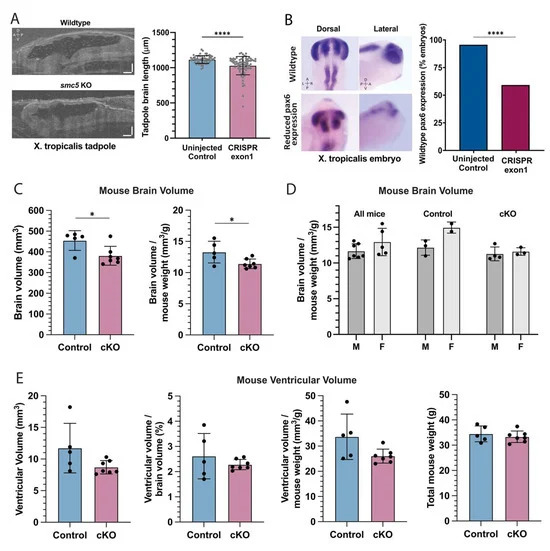
Figure 3. smc5 knockout alters brain development in frogs and mice (A) Sagittal cross-section of stage 45 X. tropicalis brain in control and smc5 KO (CRISPR exon 1) embryos. Scale bars presenting 100 m. (B) Whole-mount in situ hybridization of pax6 in stage 28 X. tropicalis embryos, highlighting reduced expression in the smc5 KO (CRISPR exon1) brain, notochord, and developing eye. Images show pax6 distribution rather than scale. (C) Brain volume and brain volume normalized by weight in control and Smc5 cKO mice measured by MRI. (D) Brain volume normalized by weight compared between male (M) and female (F) mice, showing all, control, or Smc5 cKO mice. (E) Brain ventricular volume of control or Smc5 cKO mice is shown as raw volume, ventricular volume normalized by brain volume, or ventricular volume normalized by weight. Legend: left (L), right (R), dorsal (D), ventral (V), anterior (A) and posterior (P), * p < 0.05, **** p < 0.0001 by t-test in (A,CE) and by chi-square analysis in (B).
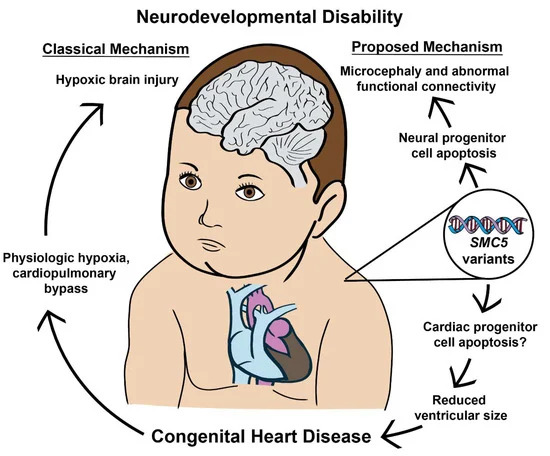
Figure 7. SMC5 malfunction during embryonic development produces CHD and NDD through independent processes. The data presented indicates that neurodevelopmental defects can occur with and without concurrent CHD. Furthermore, SMC5 mutations can alter brain FC and cause developmental delays.
Adapted with permission from MDPI Journals on behalf of the International Journal of Molecular Sciences: O'Brien et al. (2024). SMC5 Plays Independent Roles in Congenital Heart Disease and Neurodevelopmental Disability. Int J Mol Sci 2023 Dec 28;251:. doi: 10.3390/ijms25010430.
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Last Updated: 2024-02-13